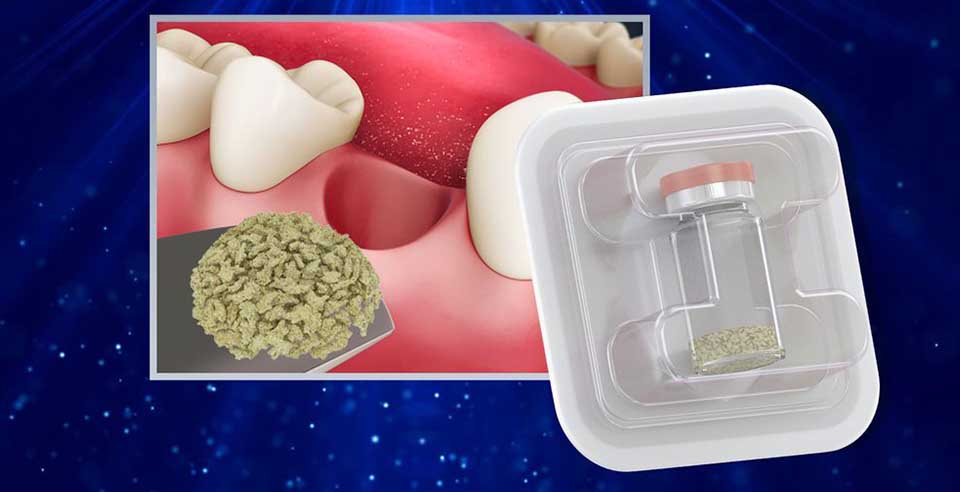
DSI Zenoss Chips are a biocompatible bone mineral matrix that is manufactured from bovine bone using a controlled and validated multistage purification process that removes all organic components. A biomaterial that is highly osteoconductive but slowly resorbed. Zenoss Chips encourage grafts that maintain structural integrity while also promoting vital bone formation over time. The presence of Zenoss chips over time stabilizes the graft, resulting in the retention of both volume and the desired shape of augmented sites. Additionally, bone mineral density is increased. As a result, grafted sites provide an ideal environment for implant survival over time. DSI Zenoss Chips' inorganic bone matrix has macro- and microscopic structures similar to human bone. Because of its trabecular architecture and interconnected macro and micropores, the implantation site promotes the formation and ingrowth of new bone. It maintains volume, consistently blocks the osteoclastic potential of periosteum and maxillary sinus mucosa (hematogenous osteoclasts), and prevents soft tissue ingrowth in the application area.
Product SKU's
| Product | Code |
|---|---|
| 1.0cc, 1000-5000 µm | ZGC40100 |
| 2.0cc, 1000-5000 µm | ZGC40200 |
Instructions
- The general principles of sterile handling and patient medication must be followed
- The filling of periodontal defects with Zenoss graft requires successful treatment of the periodontal lesion (root planing, debridement) prior to the implantation. The defect should be covered with a membrane (e.g., DSI Zenoss Bio) for optimal tissue regeneration
- After exposure of the bony defect with mucoperiosteal flap, all granulation tissue must be carefully removed
- The material should be placed in direct contact with well-vascularized, bleeding bone surfaces. The cortical bone should be mechanically perforated to facilitate the ingrowth of new blood vessels and bone-forming cells
- We suggest mixing DSI Zenoss chips with the patient’s blood or with physiological saline solution before the implantation. The chips are placed into the defect, using sterile instruments (spatula or spoon). The use of excessive force will result in the crushing of particles and loss of trabecular architecture
- In situ modelling may be performed with a sterile spatula or other suitable instruments
- Overfilling of the defects should be avoided
- It is advisable to cover the graft with a membrane barrier (e.g., DSI Zenoss Bio)
- When closing the wound, the soft tissue flap should completely cover the implanted graft and should be fixed by sutures (e.g. DSI PTFE)
- If primary wound closure cannot be achieved, further mobilization of the flap (incision through the periosteum) should be performed
- A surgical dressing may be placed over the wound for one to two weeks
- Sites grafted with Zenoss Chips should be allowed to heal approximately 6 months prior to implant placement
- A basic requirement for successful periodontal treatment includes control of any bacterial infection as well as thorough oral hygiene. It is advised that preceding the surgical intervention, there be a hygiene phase, which would include proper instruction for the patient. A postoperative maintenance phase can ensure long-term therapeutic success
Indication
- Augmentation or reconstructive treatment of the alveolar ridge
- Filling of infrabony periodontal defects
- Filling of defects after root resection, apicoectomy, and cystectomy
- Filling of extraction sockets to enhance preservation of the alveolar ridge
- Elevation of the maxillary sinus floor
- Filling of periodontal defects in conjunction with products intended for Guided Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR)
- Filling of peri-implant defects in conjunction with products intended for Guided Bone Regeneration (GBR)
Features
- Biocompatible Bone Mineral Matrix
- Osteoconductive and Slowly Resorbed
- Graft Stabilization and Volume Retention
- Increased Bone Mineral Density
- Trabecular Architecture Similar to Human Bone
- Interconnected Macro and Micropores
- Block Osteoclastic Potential
- Prevents Soft Tissue Ingrowth
Video Review
For more information