A short sentence describing what someone will receive by subscribing
Use this bar to show information about your cookie policy.
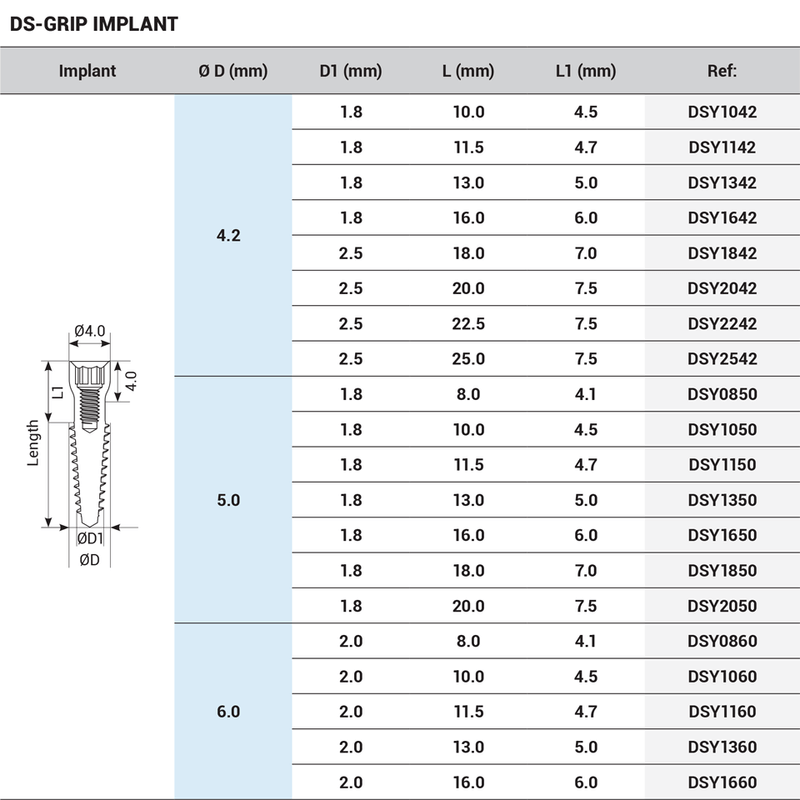
The DSI-Grip implant offers the positioning of implants at the basal/cortical bone. It can be used virtually with any bone density. It utilizes cortical or pterygoid bones, which have the least propensity to get resorption and exist even in patients who suffer from acute bone loss. It is an ideal implant for patients with bone structure problems, such as the elderly population.
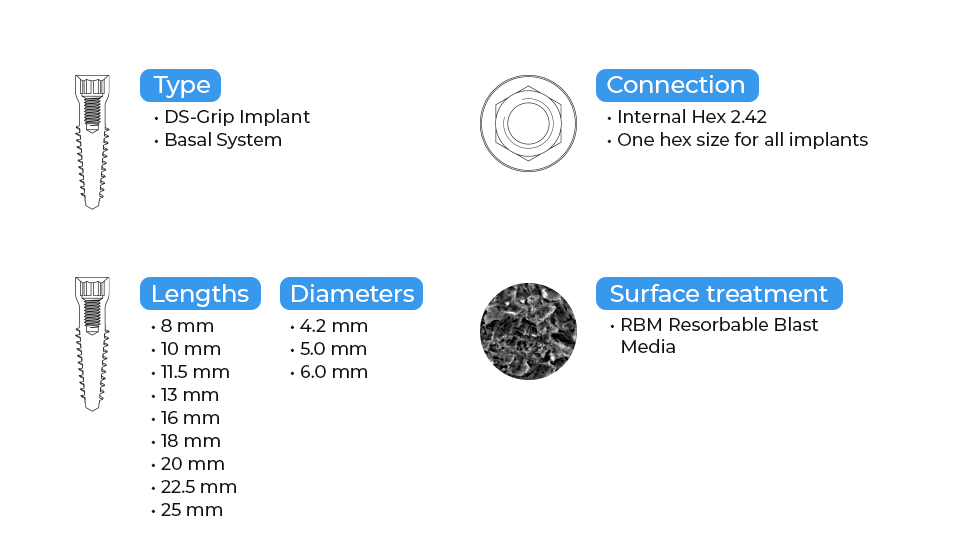
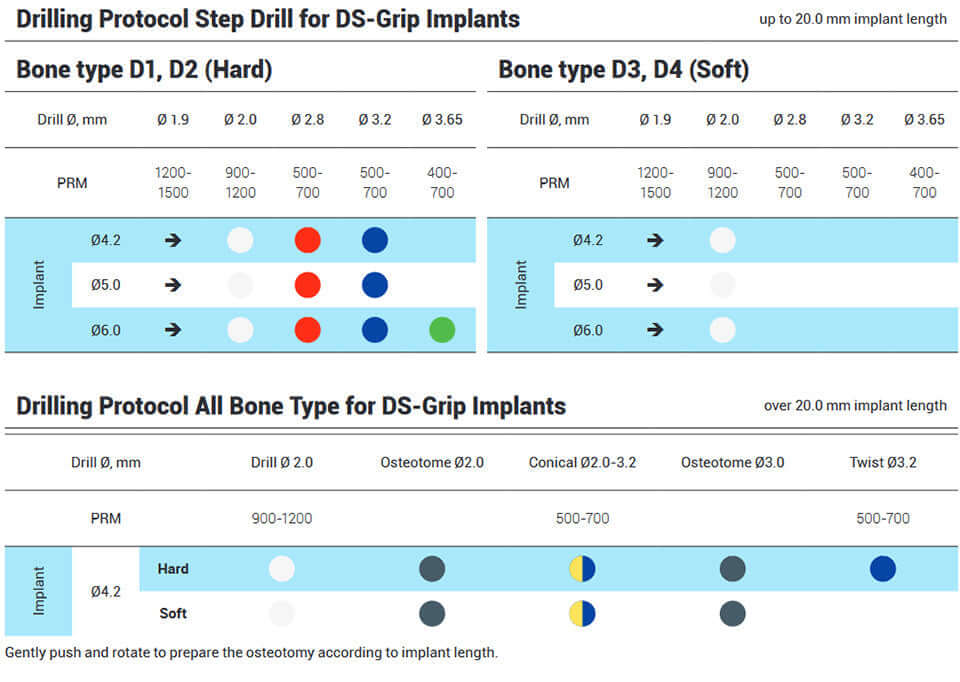
DSI Grip Implants have sharp, deep, and aggressive threads, and can be placed immediately in extraction sites without the waiting period of osseointegration or additional augmentation with bone replacement materials. Guarantees remarkable stability, together with a universal 2.42mm internal hex connection, and RBM surface treatment which increases the BIC. The smooth polished neck prevents bacterial adhesion while promoting a comfortable fit in soft tissues.
Indications:
Advantages:
RBM surface treatment:
Resorbable blast media (RBM) treatment is a process that utilizes high-speed particle blasting biocompatible calcium phosphate materials. It differentiates from traditional grit-blasting abrasives in that the implant's surface is not exposed to alumina or silicon carbide materials. In the resorbable blast material method, the excessive use of acids for particle debris removal is no longer necessary as it does not penetrate deep into the titanium surface while still delivering homogenous micro and macro porosity for the optimal implant surface topography both at cellular and protein levels. This method provides a much cleaner textured finish absent of any contaminants typically associated with other grit-blasting elements.

The DSI-Grip implant offers the positioning of implants at the basal/cortical bone. It can be used virtually with any bone density. It utilizes cortical or pterygoid bones, which have the least propensity to get resorption and exist even in patients who suffer from acute bone loss. It is an ideal implant for patients with bone structure problems, such as the elderly population.
DSI Grip Implants have sharp, deep, and aggressive threads, and can be placed immediately in extraction sites without the waiting period of osseointegration or additional augmentation with bone replacement materials. Guarantees remarkable stability, together with a universal 2.42mm internal hex connection, and RBM surface treatment which increases the BIC. The smooth polished neck prevents bacterial adhesion while promoting a comfortable fit in soft tissues.
Indications:
Advantages:
RBM surface treatment:
Resorbable blast media (RBM) treatment is a process that utilizes high-speed particle blasting biocompatible calcium phosphate materials. It differentiates from traditional grit-blasting abrasives in that the implant's surface is not exposed to alumina or silicon carbide materials. In the resorbable blast material method, the excessive use of acids for particle debris removal is no longer necessary as it does not penetrate deep into the titanium surface while still delivering homogenous micro and macro porosity for the optimal implant surface topography both at cellular and protein levels. This method provides a much cleaner textured finish absent of any contaminants typically associated with other grit-blasting elements.

We deliver worldwide.
In most cases, within 3 business days dispatch for orders - your order will be processed and prepared for delivery, one business day after receiving payment.
Please note: It is very important to write the address accurately, the package will deliver to the address you provided during the order process. Packages must be signed for by an adult aged 18+ years on delivery.
In addition to the price displayed, imported goods might be a subject of additional fees & taxes, collected by your country's customs authorities.
We accept payments via major credit cards (Visa, MasterCard, American Express...) We charge 3% Credit Cards fee.
Our website is certified by international data protection systems, and all payment pages are secured by an SSL certificate.
We accept Bank Transfers as well, please contact us for further details .
you can use the chat or whatsapp below, email or just call us.
We'll provide exact instructions on where to return/exchange your item(s).
Items must be returned in original packaging within 30 days of receipt. Exchanges may take up to 10 business days to process after we receive the tracking number.
If you don't receive your order within 30 days, you can request a full refund before the order is completed.